Steam in a piston-cylinder assembly undergoes a polytropic process. Data for the initial and final states are given in the accompanying table. Kinetic and potential energy effects are negligible. For the process, determine the work and heat transfer, each in Btu per lb of steam.
A piston–cylinder device with a set of stops initially conta | Quizlet
Feb 6, 2023VIDEO ANSWER: According to the first law of thermal dnamides, the answer to this question is that energy supplied is equal to energy stored. This can be rearranged to show that the work done is equal to the energy used and the energy supplied. Here
Source Image: askfilo.com
Download Image
5 kg of steam contained within a piston-cylinder assembly undergoes an expansion from state 1, where the specific internal energy is u_1 = 2709.9 KJ/kg, to state 2, where u_2= 2794.8 kJ/kg. During the; A piston-cylinder device contains 0.86 kg of steam at 300C and 1.4 MPa. Steam is cooled at constant pressure until one-half of the mass condenses.

Source Image: reddit.com
Download Image
SOLVED: thermodynamics As shown 5 kg of steam contained within a piston–cylinder assembly undergoes an expansion from state 1, where the specific internal energy is u1 = 2709.9 kJ/kg, to state 2, Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2, from an initial state where P1= 500 lbf/in.2, v1= 1.701 ft3/lb, u1= 1363.3 Btu/lb to a final state where u2 =990.58 Btu/lb. During the process, there is a heat transfer from the steam of magnitude 342.9 Btu. The mass of steam is 1.2 lb. Neglecting changes in kinetic
Source Image: physicsforums.com
Download Image
Steam In A Piston Cylinder Assembly Undergoes
Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2, from an initial state where P1= 500 lbf/in.2, v1= 1.701 ft3/lb, u1= 1363.3 Btu/lb to a final state where u2 =990.58 Btu/lb. During the process, there is a heat transfer from the steam of magnitude 342.9 Btu. The mass of steam is 1.2 lb. Neglecting changes in kinetic Steam in a piston-cylinder assembly undergoes a polytropic process. Data for the initial and final states are given in the accompanying table. Kinetic and potential energy effects are negligible. For the process, determine the work and heat transfer, each in Btu per lb of steam. W/m= Q/m- State p(lbf/in.2) v(ft³/lb) u(Btu/lb) 1 100 4.934 1136.
Piston-Cylinder Concept Help
Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2. The initial state is p1 = 500lbf/in^2, v1 = 1.701 ft^3/lbm and u1 = 1363.3 BTU/lbm. IN the final state, u2 = 990,58 BTU/lbm. During the process, there is heat transfer from the steam of magnitude 342.9 BTU. THe mass of the steam is 1.2 lbm. Applied Sciences | Free Full-Text | Agustín de Betancourt’s Double-Acting Steam Engine: Analysis through Computer-Aided Engineering
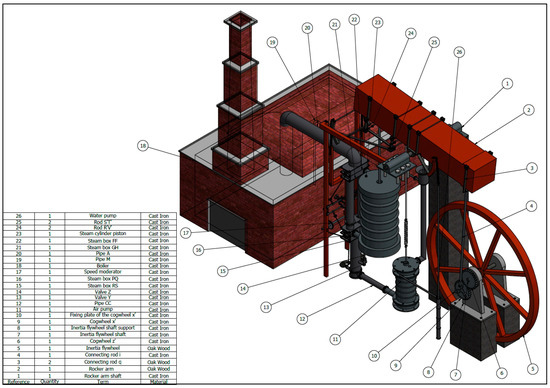
Source Image: mdpi.com
Download Image
SOLVED: As shown in in the figure below; a piston-cylinder assembly fitted with stops contains 0.1 kg of water; initially at MPa, 500 C The water undergoes two processes in series: Process Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2. The initial state is p1 = 500lbf/in^2, v1 = 1.701 ft^3/lbm and u1 = 1363.3 BTU/lbm. IN the final state, u2 = 990,58 BTU/lbm. During the process, there is heat transfer from the steam of magnitude 342.9 BTU. THe mass of the steam is 1.2 lbm.

Source Image: numerade.com
Download Image
A piston–cylinder device with a set of stops initially conta | Quizlet Steam in a piston-cylinder assembly undergoes a polytropic process. Data for the initial and final states are given in the accompanying table. Kinetic and potential energy effects are negligible. For the process, determine the work and heat transfer, each in Btu per lb of steam.
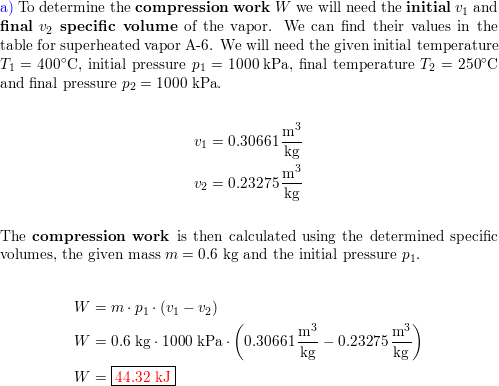
Source Image: quizlet.com
Download Image
SOLVED: thermodynamics As shown 5 kg of steam contained within a piston–cylinder assembly undergoes an expansion from state 1, where the specific internal energy is u1 = 2709.9 kJ/kg, to state 2, 5 kg of steam contained within a piston-cylinder assembly undergoes an expansion from state 1, where the specific internal energy is u_1 = 2709.9 KJ/kg, to state 2, where u_2= 2794.8 kJ/kg. During the; A piston-cylinder device contains 0.86 kg of steam at 300C and 1.4 MPa. Steam is cooled at constant pressure until one-half of the mass condenses.

Source Image: numerade.com
Download Image
Stirling engine | PDF Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2, from an initial state where V₁ = 4.38600 ft³, p₁ = 400 lbf/in², and u₁ = 1322.4 Btu/lb to a final state where u₂ = 1036.0 Btu/lb and v₂ = 3.393 ft3/lb. The mass of the steam is 2.5 lb. Changes in kinetic and potential energy can be neglected.

Source Image: slideshare.net
Download Image
JJ207 Thermodynamics I Chapter 2 | PDF Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2, from an initial state where P1= 500 lbf/in.2, v1= 1.701 ft3/lb, u1= 1363.3 Btu/lb to a final state where u2 =990.58 Btu/lb. During the process, there is a heat transfer from the steam of magnitude 342.9 Btu. The mass of steam is 1.2 lb. Neglecting changes in kinetic

Source Image: slideshare.net
Download Image
One lb of water contained in a piston-cylinder assembly, ini | Quizlet Steam in a piston-cylinder assembly undergoes a polytropic process. Data for the initial and final states are given in the accompanying table. Kinetic and potential energy effects are negligible. For the process, determine the work and heat transfer, each in Btu per lb of steam. W/m= Q/m- State p(lbf/in.2) v(ft³/lb) u(Btu/lb) 1 100 4.934 1136.
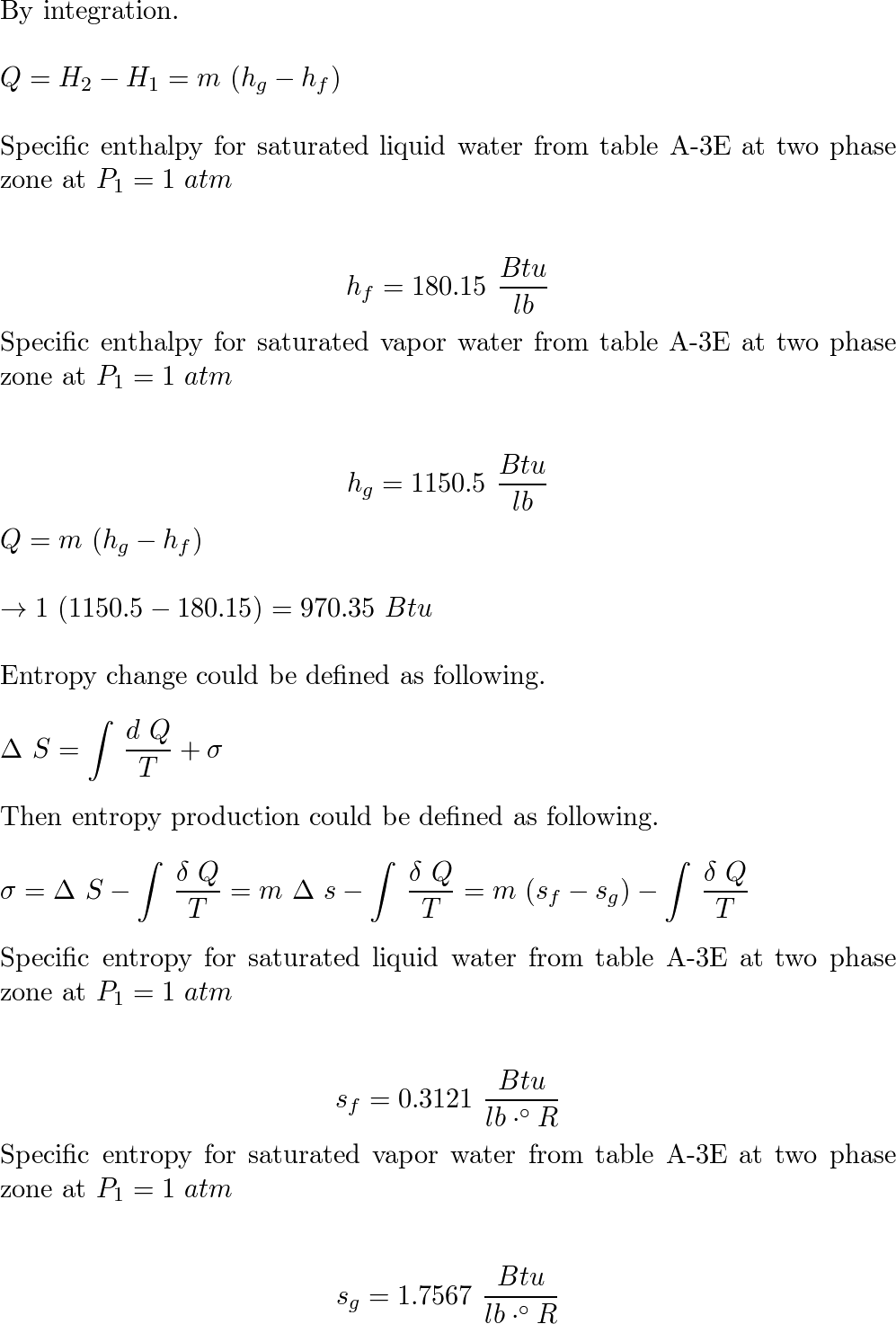
Source Image: quizlet.com
Download Image
SOLVED: As shown in in the figure below; a piston-cylinder assembly fitted with stops contains 0.1 kg of water; initially at MPa, 500 C The water undergoes two processes in series: Process
One lb of water contained in a piston-cylinder assembly, ini | Quizlet Feb 6, 2023VIDEO ANSWER: According to the first law of thermal dnamides, the answer to this question is that energy supplied is equal to energy stored. This can be rearranged to show that the work done is equal to the energy used and the energy supplied. Here
SOLVED: thermodynamics As shown 5 kg of steam contained within a piston–cylinder assembly undergoes an expansion from state 1, where the specific internal energy is u1 = 2709.9 kJ/kg, to state 2, JJ207 Thermodynamics I Chapter 2 | PDF Steam in a piston-cylinder assembly undergoes a polytropic process, with n = 2, from an initial state where V₁ = 4.38600 ft³, p₁ = 400 lbf/in², and u₁ = 1322.4 Btu/lb to a final state where u₂ = 1036.0 Btu/lb and v₂ = 3.393 ft3/lb. The mass of the steam is 2.5 lb. Changes in kinetic and potential energy can be neglected.